RECALL of CVS Health 12 Hour Sinus Relief Nasal Mist due to microbiological contamination, FDA says
CVS Health 12 Hour Sinus Relief Nasal Mist (Photo: U.S. Food & Drug Administration)
HOLLY HILL, Fla. - A CVS nasal spray is being recalled nationwide due to microbiological contamination, the U.S. Food & Drug Administration announced.
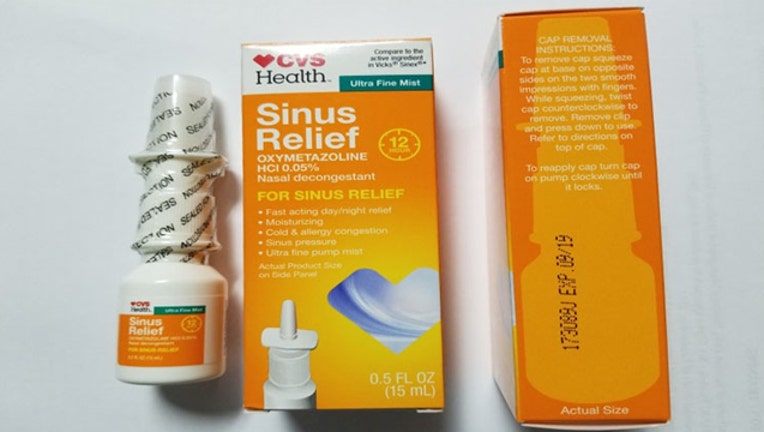
Product Quest Manufacturing is voluntarily recalling Lot# 173089J of CVS Health 12 Hour Sinus Relief Nasal Mist. The FDA says the product was found to have had microbiological contamination identified as Pseudomonas aeruginosa.
In a release, the FDA said repetitive use of a nasal spray containing a gram-negative pathogen can potentially lead to colonization and subsequent infection which can be life threatening in certain patient populations, such as those with cystic fibrosis or immuno-compromised.
Product Quest says they have not received any reports of adverse events related to this recall to the best of their knowledge.
FROM THE FDA RELEASE:
The product is used as a nasal decongestant and is packaged in a 0.5 fluid ounce bottle that is placed in an individual folding carton. 16,896 units were released with UPC code 50428432365. The affected CVS Health 12 Hour Sinus Relief Nasal Mist lot is Lot # 173089J, EXP 09/19. The product can be identified by a white nasal spray bottle and an orange label with Sinus Relief stated in white with CVS Health on top left. The IFC containing the bottle is also orange and contains the same wording. Lot 173089J and EXP 09/19 is coded on the side panel of the carton. Product was distributed Nationwide to retail outlets.
Product Quest is notifying its customers by oral and written communication and is arranging for return/replacement etc. of all recalled products. Consumers/distributors/retailers that have product which is being recalled should stop using the product and return it to the place of purchase or discard the product.
Consumers with questions regarding this recall can contact Product Quest Manufacturing LLC at (386) 239-8787, Monday through Friday from 8 am to 4 pm, EST. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
Complete and submit the report Online: www.fda.gov/medwatch/report.htm
Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration

